Exhaust gas management is arduous, and atmospheric pollution is harmful
The 2015 exhaust gas treatment task is arduous, and inventory of air pollutants that are harmful to the environment: (1) Particulate matter. The sources of particulate matter can be divided into natural sources and human sources, and human sources are the main sources. The particles that are emitted directly from the pollution source are called primary particulate matter. Particles that are generated by reactions between certain contaminant components in the atmosphere or between these components and atmospheric constituents are called secondary particulate matter. The man-made sources are mainly soot and fly ash formed during the combustion process of fuels, raw materials or product particulates discharged from various industrial processes, lead-containing compounds discharged from automobiles, and SO2 emitted from the combustion of fossil fuels converted to sulfate under certain conditions. Wait. Natural sources, such as dust from wind, waves from splashing waves, volcanic ash, burning of forest fires, cosmic dust, and plant pollen. Particulate matter is an important atmospheric pollutant. Most of the toxic substances in the atmosphere are present in the particulate matter, which is very harmful to humans, animals and plants. (2) Sulfur oxide SOx. The sulfur oxides in the atmosphere are mainly SO2, with a small portion of SO3. Mainly from the burning of fossil fuels (80% of which are coal) in power plants and heating plants, followed by smelters, sulfuric acid plants, the decomposition and combustion of organic matter, and ocean and volcanic activity. From the viewpoint of chemical thermodynamics, the equilibrium transfer rate of SO2 is high, and SO3 is easy to generate. From the viewpoint of chemical kinetics, SO3 can be generated by catalytic oxidation (ferric salt suspended in the atmosphere, magnesium salt acts as a catalyst) or chemical oxidation (mainly at the wavelength of 290-400 nm UV light), SO3 easily With water vapor generated sulfuric acid mist or sulfuric acid rain. SO2 not only has a strong irritating effect on the human respiratory tract, it also produces bleached spots on plants, inhibits growth, damages the leaves, and reduces yield. When there is particulate matter in the air coexisting, the hazard can be increased by 3 to 4 times (as described above for London smoke is an example). Many of the adverse effects of SOx are due to the sulfuric acid produced by SOx and water. The acid rain of sulfuric acid and nitric acid has seriously endangered our country and many regions of the world, and has become one of the three major global pollutions that attract worldwide attention. (3) Nitrogen oxides NOx. There are many kinds of nitrogen oxides, and the main air pollution is NO and NO2. They mainly come from the high-temperature combustion of fossil fuels (such as the combustion of automobiles, airplanes, internal combustion engines, industrial furnaces, etc.), and NO and NO2 generated by the reaction of N2 and O2 in the air (N2+O2--===2NO, 2NO+O2===2NO2) also partly comes from the exhaust gas from factories producing and using HNO3, and some production processes from nitrogen fertilizer plants, organic intermediate plants, organic intermediate plants, and nonferrous and ferrous metal smelters. Every year, NOx is released into the atmosphere by several million tons. NO can stimulate the respiratory system, but also combined with hemoglobin into nitrosyl heme and poisoning. NO2 can severely stimulate the respiratory system and can nitrate heme, which is more harmful than NO. In addition. NO2 will also destroy cotton, nylon and other fabrics, so that orange defoliation and chlorosis occurred. In addition, acid rain may also cause hazards. (4) CO and CO2. CO is the largest pollutant emitted by humans to the atmosphere, mainly from the incomplete combustion of fuel. However, recent research indicates that naturally occurring CO cannot be ignored. Due to the improvement of combustion devices and combustion technology in modern times, the amount of CO emitted from stationary combustion devices has been gradually reduced, while the amount of CO produced from the combustion of mobile sources such as automobiles is about 250 million tons per year, which accounts for the total amount of CO emitted by man-made pollution sources. About 70% of the volume. 80% of CO in urban air of modern developed countries is emitted by automobiles. The relatively abundant CH4 in the lower atmosphere can be reacted with hydroxyl radicals (–OH) to generate methyl radicals (––CH3), which in turn can be converted to CO. The ocean is another important natural source. Earlier people believed that the ocean was an important channel for absorbing CO, but it is now found that the ocean is supersaturated with CO, so that the concentration of CO in the ocean is higher than the concentration of CO in the atmosphere. The ocean emits CO to the atmosphere of about 60 million tons per year. CO is a colorless, odorless, odorless gas. The level of CO in the air of a typical city is not harmful to plants and related microorganisms, but it is harmful to humans and animals, because CO can combine with hemoglobin to generate "carbon oxyhemoglobin". The binding ability of CO to hemoglobin is 200 to 300 times greater than that of O2 and hemoglobin. Therefore, when CO enters the blood stream, the ability of the blood to deliver oxygen will be reduced or even the ability to deliver oxygen will be lost, resulting in hypoxia. Mild poisoning has headaches, nausea, and other symptoms. In severe cases, it is coma, delirium, and even death. CO2 is different from CO in that it is not toxic in itself, so CO2 is not listed as a pollutant in the past, but CO2 is also a very important pollutant in the long run. In the past more than a century, with the rapid development of industry, transportation, and energy, CO2 discharged into the atmosphere has been increasing, exceeding the ability of plants to eliminate CO2 in the natural world such as photosynthesis, and the CO2 concentration has increased rapidly. CO2 is a kind of greenhouse gas, and its increasing content will cause global warming. (5) Hydrocarbon CxHy (or HC for short). Hydrocarbons enter the atmosphere through refinery exhaust, evaporation of automotive fuel tanks, industrial production, and stationary combustion sources. A more important source is car exhaust, which always contains a considerable amount of unburned hydrocarbons unless special measures are taken to ensure complete combustion. Most of these hydrocarbons are saturated hydrocarbons (such as CH4, C2H6, C8H18, etc.), and even more serious are those in which a relatively small part of the olefins produced by the cracking of saturated hydrocarbons are relatively active, such as octane pyrolysis products of ethylene, propylene and butenes. Unsaturated hydrocarbons (45% of the vent gas) react more readily with O2, NO, and O3, producing some of the most harmful components of photochemical smog. The biological sources of hydrocarbons can not be ignored. The main emissions are CH4 and terpenoids (widely found in the leaves, flowers or fruits of certain plants). Although the release of these substances is large, they are dispersed in vast nature and therefore do not constitute direct damage to the environment and humans. However, studies have shown that increased concentrations of CH4 will enhance the greenhouse effect. Among other atmospheric organic pollutants that should also be proposed in particular, freon should be the first one to be recommended. Research shows that it is the main substance that destroys the ozone layer at high altitude.
stainless steel screen mesh is woven with stainless steel wire such as 201, 202, 304, 304L, 316, 316L etc. the weaving type for screen mesh is determined according to our customer. Such as plain weave, plain dutch weave, twill weave, dutch twilled weave, and also for material, wire diameter, mesh size, width can also be customized. The Stainless Steel Woven Mesh is anti-corrosion, wear-resistance, heat-resistance, and acid-resisting, based on this characteristics, the stainless steel woven mesh is extensively used in mining, chemical industry, food industry and pharmaceutical industry.
Stainless steel screen mesh material:
304, 316, 304L, 316L, 430, 310s etc.
Wire diamter: 0.025mm to 18mm
Weaving method: plain weave, plain dutch weave, twill weave, dutch twilled weave
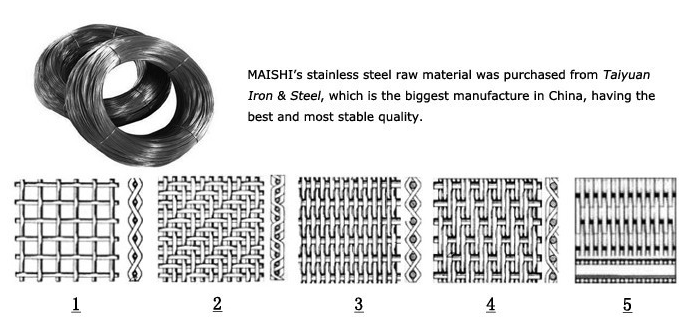
1. Plain Weave: also called tabby weave, linen weav or taffeta weave, is the most basic type of weaves. In plain weave, the warp and weft are aligned so they form a simple criss-cross pattern. Each weft thread crosses the warp threads by going over one, then under the next, and so on. The next weft thread goes under the warp threads that its neighbor went over, and vice versa.
2. Twill Weave: In a twill weave, each weft or filling yarn floats across the warp yarns in a progression of interlaces to the right or left, forming a distinct diagonal line. This diagonal line is also known as a wale. A float is the portion of a yarn that crosses over two or more yarns from the opposite direction.
3. Plain Dutch Weave: similar with plain weave, just the weft and warp wire have different wire diameter and different mesh size.
4. Twill Dutch Weave: similar with twill weave, just the weft and warp wire have different wire diameter and different mesh size.
5. Reversed Dutch Weave: difference from standard Dutch weave lies in the thicker warp wires and less weft wires.
Stainless Steel Screen Mesh Stainless Steel Screen Mesh,Stainless Steel Vibration Mesh,Stainless Steel Vibrator Mesh,High Frequency Vibrator Mesh Anping HUAHAIYUAN WIRE MESH CO.,LTD , https://www.crusherscreenmesh.com